In a multidisciplinary effort between its otolaryngology and pulmonology divisions, CHOC is now evaluating patients as young as 14 years old for a new device that could be a last-stop solution for obstructive sleep apnea (OSA), when traditional treatments have failed.
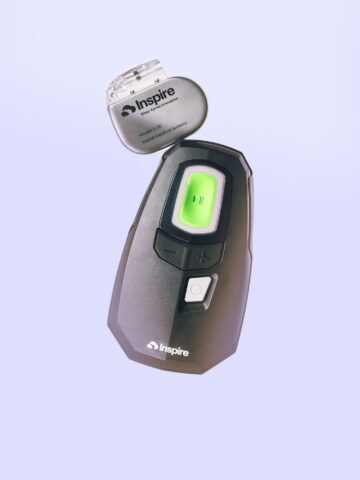
Inspire is a small device — similar to a pacemaker — that is implanted into a patient’s chest during a same-day, outpatient procedure. Inspire therapy works inside the body with a patient’s natural breathing process to open the airway with mild stimulation during sleep, allowing oxygen to flow naturally. The patient uses a small handheld remote to turn Inspire on before bed, and off when they wake up. The stimulation is very gentle and designed to move the tongue forward without disturbing the patient’s sleep. It should not be painful or uncomfortable.
“At CHOC, we believe a diagnosis, illness or injury shouldn’t put childhood on pause,” says Dr. Jay Bhatt, CHOC pediatric otolaryngologist leading the effort. “Children and teens with OSA miss out on healthy, restful sleep that is critical for staying focused at school, growing normally, and developing social relationships. This is an excellent next step for patients whose OSA didn’t respond to traditional treatments such as tonsillectomy, adenoidectomy or continuous airway pressure therapy (CPAP).”
As the first children’s hospital in California with this program, CHOC Hospital is thrilled to offer a solution to patients age 18 years old and above, who have been struggling with failed OSA treatment for years. The multidisciplinary effort is led by otolaryngologists Drs. Bhatt, Qiu Zhong and Gurpreet Ahuja and pulmonologists Drs. Chana Chin, Hanna Hong and Neal Nakra.
OSA occurs when the airway collapses during sleep and blocks the flow of oxygen to the brain. Then, the brain senses a lack of oxygen and wakes the body up just long enough to take a breath, then falls back asleep. This cycle repeats throughout the night — causing poor, disruptive sleep.
Research suggests that OSA affects up from three to five percent of the general pediatric population. For children and teens with Down syndrome, that percentage increases to 30 to 60 due to their upper airway tissues tending to be larger and more crowded.
With close to half of children with Down syndrome struggling with OSA — and most of them unable to tolerate CPAP — CHOC will have the unique position to offer a hopeful treatment to the community, as well as additional support as they transition to adulthood.
“The CHOC team is thrilled to be on the forefront of this exciting new treatment for patients with OSA,” says Dr. Chin. “We look forward to the opportunity to offer the Inspire treatment to even more patients as we expand the program in the future. We are especially lucky to offer this at CHOC’s new, state-of-the-art pediatric sleep center geared towards our most vulnerable populations with patient- and family-centered care.”
Eligible patients must be diagnosed with moderate to severe obstructive sleep apnea, cannot use or receive a consistent benefit from CPAP, and cannot be significantly obese.
The safety and efficacy of Inspire were evaluated with extensive clinical trials, and more than 18,000 implants have been placed worldwide. Both research and post-implant experience have yielded excellent outcomes, with patients using Inspire experiencing significant reductions in sleep apnea events and significant improvements in quality-of-life measures. At this time, the treatment is approved for patients ages 18 years and older by the U.S. Food and Drug Administration (FDA).
CHOC has already begun implanting devices, with more patients currently in the evaluation process. With clinical trials ongoing to expand Inspire treatment to younger children, CHOC is poised to begin offering the treatment to pediatric patients as soon as it is approved by the FDA.
Refer your patients to CHOC’s sleep disorder program