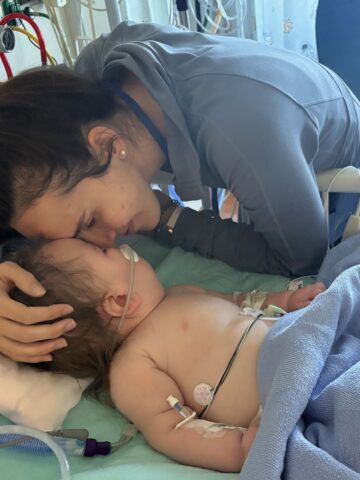
Delaware, West Virginia, Tennessee, Missouri.
Families from these and other places far from Orange County, praying for therapies for their kids stricken with rare and deadly conditions that trigger neurological declines, increasingly are turning to CHOC with little but hope for breakthrough treatments.
But in this scary and rarified world of upended lives and desperate races against time, hope is a gift – and delivering that gift is a dedicated team of metabolic and other CHOC specialists who are researching and testing gene therapy treatments often not available anywhere else.
Just this past August, for example, a nearly 2-year-old boy at CHOC became the first patient in the U.S. to receive a dose of an investigational gene therapy for the treatment of GM1 gangliosidosis, a fatal autosomal recessive disease that causes progressive neurodegeneration – and, usually, death by age 10.
“The many people on our gene therapy team are honored and delighted to be involved in this important clinical trial,” says Dr. Raymond Wang, a metabolic disorders specialist and director of the Foundation of Caring Lysosomal Storage Disorder Program.
A missing enzyme
Gene therapy involves injecting into a patient’s brain a virus that cannot replicate but instead carries a copy of a gene to produce a working enzyme. Such procedures require weeks of planning and pinpoint accuracy, with little margin for error. The goal is to get the patient to produce, on his or her own, the missing enzyme whose deficiency is slowly killing them.
Outcomes of the Aug. 27, 2021 injection of the investigational therapy manufactured by the French company Lysogene still are years away, as are other gene therapy treatments being conducted at CHOC for other rare diseases.

“We know our gene-therapy treated patients are making the missing enzymes,” Dr. Wang says. “We don’t know yet if this will reverse neurological declines over time. But these therapies are providing hope, and having hope certainly is better than sitting around watching your child decline.
“As a parent, I know what it’s like to have hopes and dreams and expectations for your children. You put yourself in these families’ shoes, and it’s really tough.”
International clinical trial
Lysogene received orphan drug designation for the treatment of GM1 gangliosidosis in the U.S. and European Union in 2017, as well as a Rare Pediatric Disease designation in the U.S. in 2016.
The CHOC patient who received the therapy in August is one of three enrolled in the clinical trial at sites in the U.S., United Kingdom, and France. He is from Missouri. Until his diagnosis, his family had never heard of GM1 gangliosidosis, for which there currently is no cure.
After some research, they discovered a nonprofit, the CureGM1 Gangliosidosis Foundation, in Albany, Ca., whose co-founder, Christina Waggoner, referred the family to CHOC.
“Dr. Wang and his team at CHOC are building momentum in seeking therapies for lysosomal storage diseases,” says Christine, whose daughter, Iris, now 13, was diagnosed at age 5 with GMI gangliosidosis. “More GM1 patients are starting to go to CHOC because of the work being done there, and that’s the way centers of excellence are built.”
Christine and her husband, Douglas, founded the GM1 foundation in 2015. At any given time, there are around 200 families active in the nonprofit.
Iris is non-verbal and can’t move independently anymore.
“She’s known for her smile and for being social,” Christine says. “She immediately makes eye contact with people. That’s her personality.”
Christine is intimately familiar with the journey GM1 families and others with kids with rare metabolic disorders are on.
“The way you survive is you don’t look back,” Christine says. “And you don’t look forward. You just try to make it through every single day.”
Fourth MPS1 patient treated
In addition to its historic Lysogene dosing for the 2-year-old patient this August, CHOC recently treated its fourth patient with the rare disease mucopolysaccharidosis type I (MPS I), a rare and progressive lysosomal storage disease also known as Hurler syndrome.
The hope is that the single-dose gene therapy – RGX-111, produced by REGENXBIO Inc. – will equip the patient’s brain cells with the information needed to make a working enzyme to stop the buildup of glycosaminoglycans in the brain.
That patient came to CHOC from Tennessee. The other three MPS I patients are from Delaware, West Virginia, and Indio. CHOC treated its first MPS1 patient, the first in the world, in October 2019.
Hurler syndrome patients are typically treated another way – with a stem cell transplant – but that poses significant risks, Dr. Wang says.
Dosings take months of planning
The dosing this summer of the patient from Tennessee was one of the most challenging yet for an Dr. Tamman Beydoun, interventional radiologist, Dr. Wang says.
Dr. Beydoun had to deftly put his access needle within a spot less than 2 mm in size — the only place it could safely go, Dr. Wang explains. The therapy was administered through a cervical puncture in the neck.
With three-dimensional visualization and guidance from a computed tomography scanner, Dr. Beydoun carefully inserted the needle into the fluid-filled space at the junction of the spinal cord and brain stem.
Then, Dr. Wang administered the gene therapy.

“I could not have asked for a better implementation of everything we had planned for many months,” Dr. Wang says. “I have so much respect for Dr. Beydoun’s work.”
Another RGX-111 dosing in late 2020 was done at CHOC intracerebroventricularly with a guidance navigation robot by Dr. Joffre Olaya from pediatric neurosurgery.
“That, too, was such a precision dosing,” Dr. Wang says. “We are continuing to make our mark as the premier site for these one-of-a-kind shots and provide hope for our metabolic families. What we’re doing is really special.”
A true team effort
Dr. Wang, Dr. Jose Abdenur, and Dr. Richard Chang are the three principal investigators (PIs) leading clinical trials related to gene therapy at CHOC. To date, there have been some 20 clinical trials.
Working closely with these PIs are Investigational Pharmacist Winnie Stockton; Metabolic Research Coordinators Nina Movsesyan, Ingrid Channa and Harriet Chang; and Investigational Pharmacy Technician Harumi Hope.
Winnie, assisted by Harumi, makes sure investigational drugs for gene therapy are received and stored properly, and explains to patients the immune-suppression drugs they use post therapy.
Investigational drugs involve detailed preparation, storage, and handling and the timing of the procedure and drug preparation must be well-coordinated, says Winnie, who prepares them in the inpatient pharmacy at CHOC.
“It’s really exciting making medicine breakthroughs and making these options available to families,” Winnie says. “I find investigational drugs really interesting because I have an opportunity to see the drugs being developed that could become the standard of care for future patients with rare diseases.”
Working hand in hand with patients
Nina manages the metabolic research coordinators, supervising and coordinating all the clinical trial studies in the metabolic division of the CHOC Research Institute.
The research coordinators work closely with families, handling everything from scheduling to arranging for temporary housing while they are in Orange County. They also serve as the point of contact between the PIs and patients, as well as companies like Lysogene who sponsor the clinical trials.
“We basically hold the hands of each patient and their families from one appointment to another,” Nina says. “It’s huge when members of our team, including the physicians who are so busy, express their support for us.”
Adds Nina: “Sometimes, our jobs can be emotionally overwhelming when a patient passes away. But everyone is so excited to contribute to this research. For every one of these families, providing hope is our goal.”
Time is of the essence
Ingrid is one of the newest members of the metabolic research coordinator team, having been at CHOC for a year. She is working with the GM1 study and helps coordinate a compassionate use program for a drug pending approval from the U.S. Food and Drug Administration.
“It’s unimaginable what families go through,” Ingrid says. “We are all aware of the severity of the conditions and the potential prognosis, so we try very hard to make things happen as soon as possible, because every day counts.”
Adds Ingrid: “It’s very meaningful to work with these families and rewarding to get to know them, and exciting to be part of a team that is working on such a valuable cause like this.”
Says Dr. Wang: “I’m so grateful for such an awesome team of people working together for one common goal.”
A child’s legacy
Christine, co-founder of CureGM1, believes her foundation has contributed to moving the needle in helping GM1 families.
Her husband, an animator and roboticist, now works part-time to help take care of Iris.
The couple has a son, Carter, 11, who is healthy.
Iris’ mother describes her as a strong and sweet girl. She loves wrangling with her little brother on a regular basis. She loves riding horses and swim therapy and attending school.
“The hope is that there is hope,” says Christine. “And hopefully, that will be my daughter’s legacy.”
Learn about pediatric research and clinical trials at CHOC