In 2021, Rady Children’s Hospital Orange County, formerly called CHOC, became the first children’s hospital in California to introduce a novel therapy for adolescents with obstructive sleep apnea (OSA), starting with ages 18 and older and expanding to 13 and up for patients with Down syndrome following FDA approval.
A little more than four years later, the Inspire device, a nerve stimulator that works somewhat like a pacemaker, has been implanted in 17 Rady Children’s patients – more than any other pediatric hospital in the state. The device is for patients who can’t tolerate traditional treatments like CPAP (continuous airway pressure therapy), which requires a person to wear a mask attached to a tube while sleeping to treat OSA, the most common type of sleep apnea.
Now, as one of five approved pediatric healthcare centers nationwide that are part of a post-FDA (Food and Drug Administration) study to assess the effectiveness of the Inspire device, Rady Children’s is poised to see a significant increase in the number of patients to undergo the same-day, outpatient procedure.
The device has a proven safety record in adults, with 99.6% of Inspire implant procedures being successfully completed without any major complications, and 93% of these adults recommending the therapy.
Since fall 2025, Rady Children’s been participating in a multicenter post–FDA-approval research study. Results are expected in several years.
The Inspire team at Rady Children’s is a joint collaboration with the ENT surgery and pediatric pulmonology sleep teams, led by Dr. Jay Bhatt and Dr. Qiu Zhong from ENT and Dr. Chana Chin from pulmonary sleep medicine. Dr. Neal Nakra, medical director of pediatric pulmonology, and Dr. Hanna Hong also comprise the Sleep team, and Dr. Ethan Muhonen will be joining the ENT team soon.
“The Inspire device has made a huge difference for our patients,” Dr. Zhong says.
Dr. Chin started the Inspire program in 2015 after learning about the device while undergoing her medical training at USC Keck.
“We were looking for other alternatives to PAP, as the entire sleep world is,” Dr. Chin says. “For some, PAP can be hard to wear and use. Given how difficult PAP can be for some of our pediatric patients, we knew that Inspire could be a great alternative for some of our select patients with OSA.”
Higher risks
Between 30% and 60% of children and teens with Down syndrome have OSA due to their upper airway tissues tending to be larger and more crowded. They also have neurological reasons for being more prone to the condition compared to the general population.
People with OSA may be at a higher risk for serious health concerns if left untreated like type 2 diabetes, stroke, heart attack, depression and a shortened life span.
The implant is designed to be a long-term, mask-free solution for people with OSA who have tried and struggled with CPAP.
Approved by the FDA for use in all patients 18 and above in 2020, the FDA gave the green light for the implant to become available for patients with Down syndrome at least 13 years old in 2023 (the agency has yet to approve it for other pediatric patients).
Since then, Rady Children’s has been a leading proponent of the device, manufactured by Inspire Medical Systems, Inc. of Golden Valley, Minn.

How it works
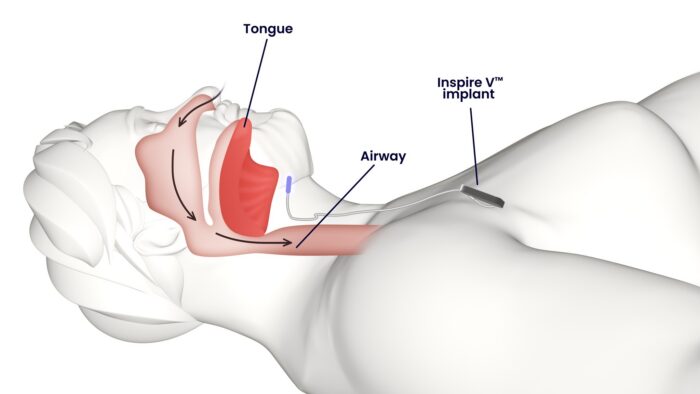
OSA occurs when the tongue relaxes back into the airway while a person sleeps, causing the airway to close. This causes a person to wake up and gasp for air.
Therapies like CPAP use a mask and hose to force air through the airway.
The Inspire device, which is implanted above the nipple area right below the collarbone, works inside the body and syncs with a person’s natural breathing.
A person activates the device when he or she goes to bed via a wireless remote.
As it monitors their breathing, the device can tell if the user is taking a breath or if there is an obstruction.
When it senses that the user is not taking a big enough breath or if there is a blockage, it sends an electrical signal to the hypoglossal nerve, which is involved in controlling tongue movements required for speech and swallowing.
A gentle pulse travels from a wire under the skin up into the neck and to the hypoglossal nerve, causing the tongue to stiffen and protrude, unblocking the back of the throat to allow for normal breathing.

Other criteria for treatment
Dr. Chin and her colleagues note that patients have to meet strict criteria to be considered as candidates for the Inspire device.
For example, kids with Down syndrome have to register a score of at least 10 on the apnea-hypopnea index (AHI), which measures the average number of times a person stops stop breathing (apneas) and has shallow breathing events (hypopneas) per hour of sleep.
They also have to undergo a sleep endoscopy to confirm that they haven’t experienced complete concentric collapse at the soft palate — and of course, be unable to use or benefit from CPAP.

A multidisciplinary model
Dr. Bhatt calls the Inspire device a “particularly impactful option” for Rady Children’s patients with Down syndrome.
“We’ve seen meaningful and sustained improvements in sleep-related symptoms, including reductions in apnea burden, improved sleep quality, and better daytime functioning,” Dr. Bhatt says. “For patients with Down syndrome and their families, this has often translated into noticeable gains in energy, attention, behavior, and overall quality of life after years of limited effective treatment options.
“The success of the program has relied heavily on a multidisciplinary model involving ENT, pulmonology, sleep medicine and close family engagement to ensure appropriate patient selection and follow-up,” Dr. Bhatt adds.
“Overall, Inspire has made a substantial difference in the care of patients with Down syndrome and refractory obstructive sleep apnea, expanding both treatment options and long-term outlook for these families.”