For anyone not trained in medicine, let alone a child, the title of the research study is a lot to grasp:
“Pharmacogenomics to characterize interactions between vincristine and triazole antifungals in cancer patients.”
But kids who agree to participate in a research study first must provide assent. This process includes reading and agreeing to what’s written on an assent form, indicating they understand the study, what they’re required to do, and what they can expect to have happen to them as a result of their participation.
To make things easier for children and their families, CHOC has rolled out an eye-catching comic book format to explain research projects to children ages 7-11 and request their participation.
The Comic Assent project, the brainchild of Kirsten Kasper, a clinical research coordinator in CHOC’s Research Institute and who supports investigator-initiated research conducted by oncologists in the Hyundai Cancer Institute at CHOC, is growing in use since launching in March 2022 – and it could catch on as a model for pediatric healthcare systems everywhere, according to its creator and clinicians.
The above-referenced study of antifungals in cancer patients, led by Dr. Martin Tuan Tran, an infectious diseases pharmacist at CHOC, comes with a three-page assent form packed with colorful explanatory cartoons and more easy-to-understand explanations, such as:
The doctors want to learn more about genes that control enzymes that break down medicine in your body.
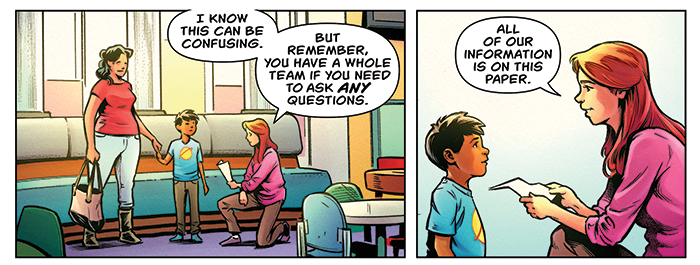
One cartoon panel shows a child and his mother talking to a CHOC research coordinator.
“So, I can change my mind, and no one will be mad?” the boy asks about his participation in the clinical trial.
“That’s right,” the research coordinator responds. “No one will be mad.”
The assent form explains that one swab will collect cells from the inside of the cheek, and that you can’t eat or drink 30 minutes before the swab.
“It’s a little tickle,” the form says of getting swabbed. “Try not to laugh!”
Art and research
Kirsten majored in biology from Cal State Fullerton and, before joining CHOC in 2018, she worked at UC Irvine, first as a unit secretary and then in data management and research at the UCI Health Chao Family Comprehensive Cancer Center.
She came up with the idea of the Comic Assent after recognizing the need for young patients to better understand research studies. For clinical trials and research studies, parents must give their consent to kids under 18 years of age as children are not typically able to provide informed consent.
The Comic Assent was made possible by a CHOC Foundation One Wish Grant, a philanthropy-supported fund for programs pitched and developed by CHOC clinicians and associates to improve the patient and family experience.

The support of Keri Zabokrtsky, manager of health sciences administration in the CHOC Research Institute, was critical to getting the Comic Assent launched, Kirsten says.
“This would not have happened without her,” she says. “She is a champion and an amazing grant writer.”
Says Keri: “I am thrilled that Kirsten took the initiative to bring this important project to life. This all came about after I shared with Kirsten complaints lodged by a researcher about the complexities of the informed consent document, and we started to discuss how illustration may be used to better convey medical information. Not only do we expect it to benefit young children but also, indirectly, adolescents and parents.”
Putting together a team
Kirsten initially looked for illustrators who might be able to service the One Wish Grant project. She and the One Wish Grant team then narrowed the field to Lancaster, Penn.-based Jason Piperberg, who created 26 images that were honed with the input of 25 children to make the illustrations as effective and relatable as possible.
Dr. Michelle Miller-Day, a professor of Communication Studies at Chapman University working as a focus group consultant on the Comic Assent project, helped interview children for their input and refine the final wording.
The Institutional Review Boards (IRBs) at CHOC reviewed and suggested changes to the Comic Assent throughout the development process. The final iteration is available as a template for all research projects.
One critically important aspect of research is that participation is voluntary, notes Megan Bailey, director of the office of research compliance at CHOC.
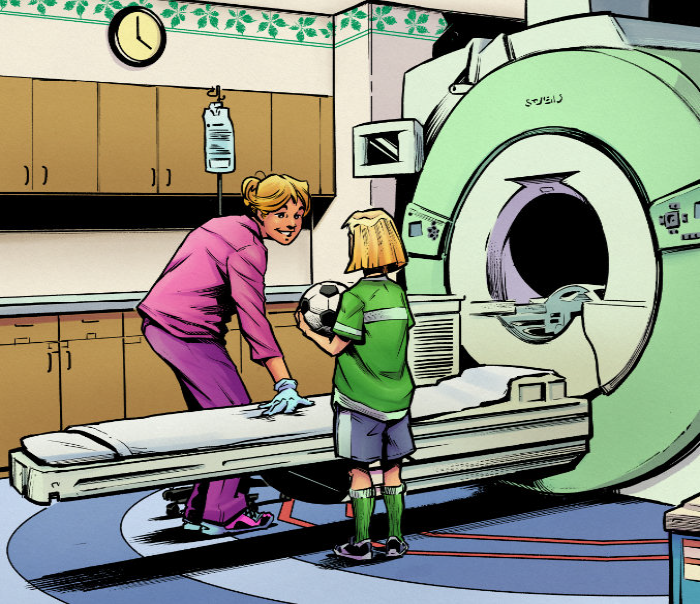
“California regulations recognize that school-aged children, starting at the age of 7, deserve the autonomy to provide or withhold their assent to participate in research,” Megan explains. “But how can kids make an informed decision without information that makes sense to them?
“Just as picture books convey information to this age group, the Comic Assent conveys information to our elementary school-aged children to help them make decisions for themselves.”
Unique to the Comic Assent are dialogue boxes and panels that can be tweaked to depict procedures involved in virtually any research project, Kirsten says.
“I have only seen two other hospitals use comics but those were for standalone studies,” she adds. “The magic of ours is that is customizable.”
The comic can be customized for other clinical trials
Dr. Tran is a big fan.
“I think it’s a great idea,” he says. “I’m actually surprised nobody has thought about this until now. I think it’s very helpful to have something colorful with pictures to go with all the verbiage that are in assent forms.”
In addition to Dr. Tran, pediatric oncologist Dr. Lilibeth Torno (some illustrations were modeled after her likeness) used the Comic Assent for her study, “Use of serum brain-derived neurotrophic factor (BDNF) to evaluate neurocognitive decline and recovery in pediatric, adolescent, and young adult (AYA) patients newly-diagnosed with acute lymphoblastic leukemia (ALL) or lymphoblastic lymphoma.”
Pediatric oncologist and stem cell transplant Dr. Rishikesh Chavan is using Comic Assent in an active study titled “Role of regulatory T cells in predicting outcomes of stem cell transplantation,” with plans to use it in future clinical trials, too.
“Comic Assent is really an innovative and unique way of explaining to youngsters what specific research questions they are being consented for and how their choices impact them and others who may benefit from the research,” Dr. Chavan explains. “It not only ensures better communication but also helps protect against health literacy bias by making it easier for all participants from diverse backgrounds to understand what they are signing up for.”
Meanwhile, Jessica Ardo, a supervisory clinical research coordinator, is using the Comic Assent for a study involving patients in the Mental Health Inpatient Center (MHIC). She used the Comic Assent with patients who were adjusting to being admitted and the milieu of the unit, with patients who were on the autism spectrum, and patients who were admitted for suicidal ideation or attempt.
“I found it helpful, and some patients expressed liking the pictures,” Jessica says. “Some parents also were reassured that the Comic Assent was presented to their children as another way of giving them information.”
Adds Jessica: “Kirsten’s work has been important to our work as coordinators speaking with children about research studies and helping them understand what it is we are asking of them. We know being in the hospital can be stressful and overwhelming to children and their family members, and they are given a lot of information when they are here.
“Presenting some of this information in a graphic, picture format can help make it easier to have a conversation about research. I work in psychology and psychiatry and our patients are sometimes also having mental and behavioral health struggles. Having a tool like Kirsten’s gives us another way to engage with children and their family members and answer questions they may have.”
Comic Assent is available in many languages
Kirsten is reaching out to other research coordinators to see what they need in future versions of the Comic Assent.
She says the concept has been particularly useful to patients with cancer and their families.
“Especially for families seen in the Hyundai Cancer Institute, where their world has been turned upside down. When they’re presented with the opportunity to participate in research studies, making the assent form as clear and understandable as possible helps a lot,” Kirsten says.
Comic Assent is available in English, Spanish, and Vietnamese, with versions in Arabic, Korean, and Chinese coming soon, Kirsten says.
Future studies will gauge the effectiveness of Comic Assent, she adds.
After that, the sky’s the limit.
Learn about pediatric research and clinical trials at CHOC