Learn how the CHOC Foundation of Caring Lysosomal Storage Disorder Program and Metabolic Lab are advancing lysosomal storage disorder treatment.
CHOC receives prestigious NIH grant to study treatment for rare lysosomal storage disease
Grant’s support will help advance gene-therapy treatment for the rare lysosomal storage disease mucopolysaccharidosis type I (MPS I).
Rare mitochondrial diseases are the focus of Feb. 10 virtual CureARS event
The upcoming webinar is open to the public, and will share the latest developments in the battle against rare mtARS mitochondrial diseases.
CHOC conducts clinical trial for Glycogen Storage Disease type 1a
CHOC selected as West Coast site for gene therapy clinical trial for patients 8 years and older with Glycogen Storage Disease type 1a.
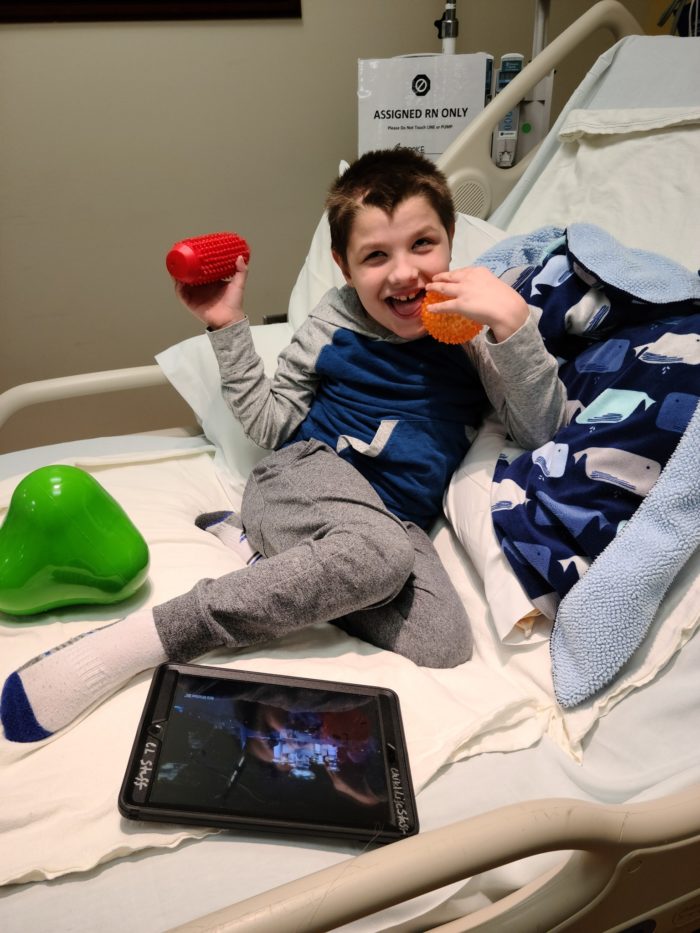
Enzyme replacement therapy slows progression of CLN2 Disease
CHOC is at the forefront to offer breakthrough enzyme replacement therapy to improve the quality of life for children with CLN2.
Batten disease patients highlight CHOC’s growing reputation as a destination for kids with rare conditions
Children from throughout the U.S. travel to receive CLN2 treatment at CHOC, the largest Brineura infusion center in the nation.