Their first date was a trip to the grocery store.
Ben had wanted to take Rachel out for pizza.
But when she told him about the rare disease she has that drastically limits what she can eat and requires her to ingest cornstarch every four hours around the clock, they agreed they should buy food together.
“I pointed out to him the things I could eat and how to read ingredients,” Rachel recalls.
The date was a success. Six months later, Ben proposed to Rachel.
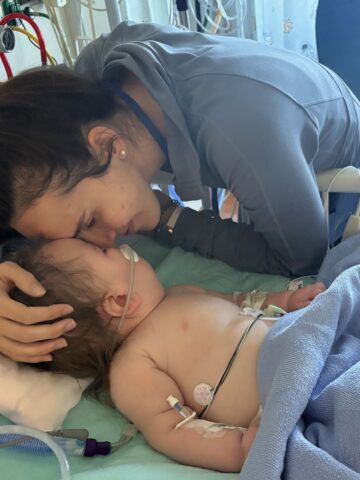
Now, 10 years after she and Ben wed, Rachel Wengel is making history at CHOC.
In July 2022, she became the first patient here (and sixth patient worldwide) to participate in the phase 3 investigational gene therapy for the treatment of Glycogen Storage Disease type 1a (GSD1a), the most severe genetically inherited glycogen storage disorder.

Because of a defective gene, Rachel’s body can’t give proper instructions to an enzyme that is involved in the process of breaking glycogen down to make glucose for the body to use as energy.
Without a careful diet and regular ingestion of cornstarch, people with GSD1a can quickly develop severe hypoglycemia (low blood sugar), causing seizures, coma, and potentially death, and they are at risk for long-term complications including gout, anemia, renal failure, kidney stones and liver tumors.
Phase 3 clinical trial for GDS1a
CHOC has been selected as the only West Coast site for the phase 3, randomized, double-blind, placebo-controlled gene therapy study for patients 8 years and older with GSD1a.
The clinical trial will have an estimated enrollment of 50 participants and will involve 29 hospitals around the world (with Utah and Colorado being the closest sites to CHOC). The industry sponsor of the study is Ultragenyx Pharmaceutical Inc.
Although Rachel is an adult, CHOC aims to enroll pediatric patients soon in the clinical trial.
Participants will be closely monitored during the two-year study by specialists in CHOC’s Division of Metabolic Disorders, including division chair Dr. Jose Abdenur, director of the metabolic laboratory at CHOC, Monica Boyer, a certified pediatric nurse practitioner, and Mary Sowa, a clinical dietician.
The three say that monitoring and caring for GSD1a patients like Rachel is a huge team effort that also involves specialists in CHOC’s pharmacy, resource coordinators, highly trained nurses and others.
“It’s a very involved effort that involves multiple disciplines throughout CHOC,” Monica says.
“This study and other ongoing gene therapy trials at CHOC are examples of our commitment to help families with rare diseases,” Dr. Abdenur adds.
Money in the bank
In the clinical trial, clinicians will use a naturally occurring virus that will travel through the bloodstream to the liver to provide the corrected version of the G6PC gene, resulting in the production of a normally functioning G6Pase enzyme. The goal is for the study participant to be able to properly break down glycogen to make glucose – something that happens naturally in healthy people, allowing them to fast for hours with no issues.
Monica uses the analogy of money in a banking account.
“Healthy people can store glycogen in their liver and use it for withdrawal later when they need it, but people with GSD1a are unable to break glycogen down for future use,” she explains. “They are unable to make that withdrawal from the bank.”
Adds Dr. Abdenur: “This investigational gene therapy jumpstarts the body’s glucose control. If it works, this therapy will result in a remarkable change in terms of these patients being able to experience a successful improvement in their quality of life.
“Even a 50 to 70 percent reduction in the consumption of corn starch will be a huge improvement for these patients.”

Results from earlier phases of the clinical trial were promising, Monica notes.
In those studies, patients were able to reduce their daily cornstarch intake by approximately 75 percent, and some patients were able to come off starch completely, she says.
“Will these patients be able to fast longer with this investigational therapy or reduce the amount of starch that is required for their daily needs?” Monica asks. “This study will be able to answer those questions.”
Rachel’s GSD1a, NG tube and cornstarch
GSD1a is rare, with an incidence rate of 1 in 100,000 in the general population.
“We’ve known about this disease since around the 1930s,” Dr. Abdenur says. “It’s one of the oldest metabolic diseases to be described, but we haven’t yet been able to provide any better therapies.”
Rachel, who grew up in Idaho, was diagnosed with GSD1a at around two months old. Her older brother, Hyrum, also has the disease.
Doctors started Rachel on cornstarch when she was a baby and she had to wear a nasal gastric (NG) tube that would deliver liquid food while she slept. At age 3, she started putting in the NG tube herself.
When she was 15, Rachel stopped using the NG tube at night and woke up every four hours to ingest cornstarch dissolved in water.
“It was definitely a struggle,” she says of growing up with GDS1a.
She couldn’t eat sugar, dairy, or fruits. Since about half of her caloric intake was starch, her other food selections were limited to meat, salads, and grains.
Despite her disease, Rachel managed to have two healthy children with Ben: Echo, 6, a girl, and Michael, 4.
Her CHOC clinicians say that’s remarkable for someone with GSD1a. Management of GSD1a during pregnancy is very challenging. Carbohydrate requirements change during pregnancy and by the end of Rachel’s pregnancy, her cornstarch intake had nearly doubled and she was eating almost every hour.
CHOC’s team approach to care
Rachel moved to California in 2011 and started seeing CHOC specialists in 2013.
“What’s really great is the team approach at CHOC,” Ben says. “They always ask us, ‘How can we better serve you?”
Rachel’s CHOC team has come up with new ways to make it easier for her to manage her disease.
For example, they recommended a continuous glucose monitor so she wouldn’t have to prick her thumb to check her blood sugar. And CHOC got her on a new product, Glycosade, which allows her to go six hours between starch doses.
Mary has helped Rachel with recipe recommendations to give her more energy and to jazz up her diet.
“This is how being a doctor should be: finding solutions to patients’ daily lives,” Ben says.
Says Rachel: “Everyone here is super kind.”
To participate in the clinical trial, Rachel had to demonstrate stability for a minimum of four weeks. This information provided good baseline data for comparison during the actual study period, such as how long she could fast between starch doses.
“Rachel has been waiting for something like this study her whole life,” Mary says. “And she was so wonderful during the lengthy screening process.”
Says Rachel: “I’m super excited.”
For more information about the GSD1a clinical trial, visit clinicaltrials.gov and search for study DTX401-CL301
Refer a patient to CHOC’s Division of Metabolic Disorders