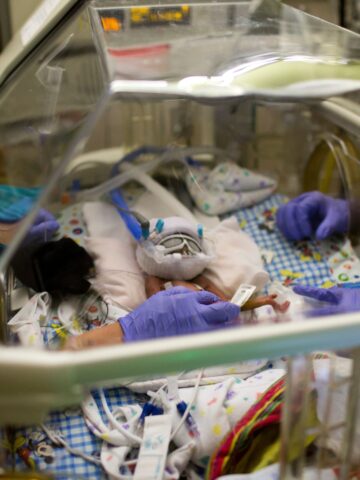
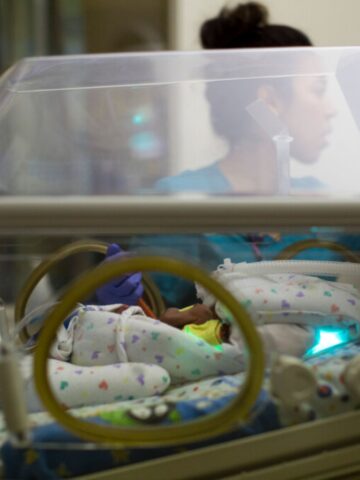
Multi-site clinical trial tests the efficacy, safety and pharmacokinetics of Lacosamide
For more than a year, pediatric neurologist Dr. Simon Kayyal, working with research coordinator Angelyque Lorenzana, meticulously plotted out a course of action should a patient meet the criteria needed to enroll a patient in a clinical trial for an investigational drug to treat neonatal seizures.
A couple of days after he was born on Sept. 21, 2022, Christian LeMaster would become that candidate.

Study of Lacosamide efficacy for treatment of neonatal seizures
Lacosamide has been approved for the treatment of partial onset seizures in children 1 month or older. Participating in a multi-site clinical trial spanning 14 states, Australia and Canada, the goal of the industry-sponsored study is to test the efficacy, safety and pharmacokinetics of Lacosamide in the treatment of neonatal seizures (children less than 1 month of age).
Dr. Kayyal, who has a special interest in neonatal neurology and who has spent considerable time in the neonatal intensive care unit (NICU) at CHOC since starting here in October 2019, signed up as principal investigator of the industry-sponsored clinical trial, of which CHOC is one of six participating sites in California.
Part of his work included the creation of a workflow that defined the roles and responsibilities of himself, research coordinators, neonatologists, epileptologists, research pharmacists, EEG technicians and nursing staff that would care for patients participating in the study.
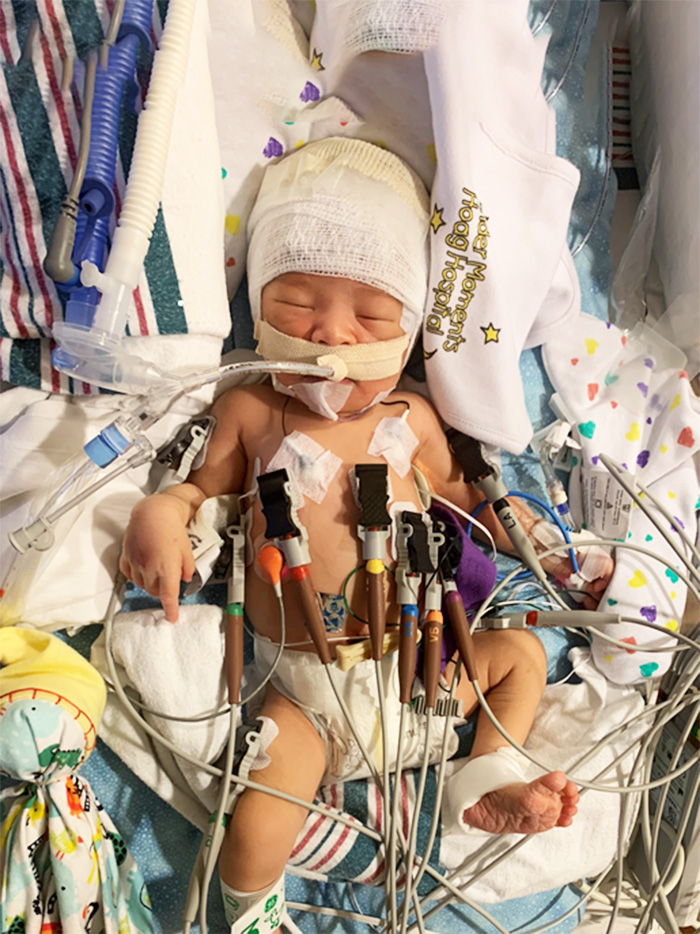
After many mock screenings and enrollments, that workflow was finalized and would be put to the test.
But before the well-rehearsed process could begin, Angelyque had to secure consent from Christian’s parents, Patrick and Briana LeMaster, prior to Christian being able to participate in the study.
Complicating this delicate and nuanced process was the fact that Briana still was at the birthing hospital, following a Cesarean section.
The stars aligned
Launched in March 2021 and expected to be completed in September 2023, the clinical trial in which Dr. Kayyal serves as a principal investigator for is called the LENS study.
The study is designed to measure whether Lacosamide is effective as a second-line treatment of neonatal seizures compared to traditionally used second-line treatments (a second-line treatment is one that is given when a child is still having seizures despite having received first-line treatment options).
When enrolled, a patient who meets the criteria for the study will be randomized to either receive Lacosamide or traditionally used medication (such as fosphenytoin) as a second-line treatment.
Published data for the treatment of neonatal seizures is sparse in terms of the most efficacious medications, Dr. Kayyal notes.
“Rigorous studies of these kind are unfortunately limited but necessary to better ascertain which anti-epileptics are optimal for the treatment of neonatal seizures,” he says. “We have some data on optimal first-line anti-epileptic therapy – however, the data for optimal second-line therapy is still insufficient.”
In addition to informed consent being provided, participants in the study had to have received first-line treatment and have had a specific duration or number of seizures prior to enrolling into the study (amongst several other criteria).
“The stars really had to align in order to potentially enroll Christian,” Dr. Kayyal says.
Upon meeting the necessary criteria for enrollment and upon randomization, Christian received Lacosamide during the treatment period – a process that required a lot of pre-planning with CHOC Investigational Pharmacist Dr. Winnie Stockton.
Dr. Stockton created an electronic order that would facilitate research medication delivery to Christian in the necessary two-hour window after the onset of seizures.
“When a patient is being treated for seizures,” Winnie says, “that’s something clinically we want to address very quickly.

“In research you want to allow adequate time for the family to consider participating and to be informed about the clinical trial, but you don’t want to delay treatment,” she adds.
The documentation for the administration of investigational drugs is above and beyond what normally has to occur.
“For this study,” Winnie says, “we built some electronic processes and established a plan for communication with the pharmacy to ensure a smooth workflow and facilitate documentation.”
Advancing pediatric research at CHOC Research Institute
Clinical trials are the engine that drives CHOC’s Research Institute to go beyond to provide the best possible treatment for patients.
“We never forget at CHOC that our patients and families are front and center with everything we do, and that’s certainly true of research,” says Brent Dethlefs, executive director of the CHOC Research Institute. “We look at questions that arise from the bedside and bring solutions back to the bedside.”
And parents are the key to having their non-adult child participate in clinical trials through the consent process.
The LeMasters, despite the trauma of dealing with a critically ill newborn – their first – realized the importance of advancing pediatric research. They said the compassionate, clear, and informative approach by Dr. Kayyal and Angelyque in seeking their consent was key to their decision to proceed with the investigational medication for Christian.
“As you can imagine,” Patrick says, “this was overwhelming as new parents. All of this happening in such a short amount of time was scary. But after talking to my wife, we agreed that if this could be happening to other kids down the road, if our son could provide some insight into this, I think it would be a good thing to do.”
Dr. Kayyal says his and Angelyque’s goal was to make the LeMasters feel educated enough to make an informed decision.
“We never felt they were pressuring us,” Briana says. “The staff at CHOC was incredible. They told us, ‘Be with your child. We want to make you comfortable.’ They brought so much hope in our life in such a tough situation. I just can’t praise them enough. They put so much love and compassion into their work.”
Doing well
Christian, at 4 months old, is doing well, the LeMasters are happy to report.
So far, he’s had no more seizures.
“He’s doing everything normally that a baby would be doing at his age,” Patrick says.
The LeMasters say they’re happy with their decision to enroll their newborn in the LENS clinical trial.

While at CHOC, “Not only did they care of our son,” Patrick says, “but also us as parents. They helped manage our expectations so we could celebrate the small wins. And one thing that stuck with us is how we were told progress doesn’t always come in a straight line.”
Says Dr. Kayyal: “This study will help us make an assessment as to whether or not there is a place for Lacosamide in the neonatal population. Clinical trials like this are essential in pushing the field of neonatal neurology forward.”
Learn about pediatric research and clinical trials at CHOC