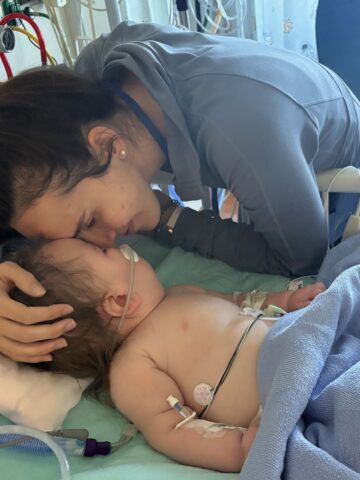
In a prestigious win for the CHOC Research Institute, Dr. Raymond Wang has been awarded a $3.2-million National Institutes of Health (NIH) research project grant (R01) from the Eunice Kennedy Shriver National institute of Child Health and Human Development to advance gene-therapy treatment for the rare lysosomal storage disease mucopolysaccharidosis type I (MPS I).
An NIH R01 grant is considered a holy grail for medical researchers. The federally funded grants are extremely competitive, with fewer than 10% of applicants receiving them.
Dr. Wang, a metabolic disorders specialist and director of CHOC’s Foundation of Caring Lysosomal Storage Disorder Program, is principal investigator of the multi-center study supported by the grant.
The five-year study aims to pave the way for eventual treatment for humans with MPS I who suffer from debilitating joint pain and degeneration following stem cell or enzyme replacement therapy that is effective for treatment of neurologic disease, but not orthopedic disease.
Stem cell transplant treatment for MPS I patients must be done as early as possible to prevent neurodegeneration, but can be risky and often leaves children with severe deficits performing activities of daily living.
The NIH study, which began May 1, 2023, will involve different combinations of gene therapy on the canine animal model of MPS I in collaboration with Duke University and the University of Pennsylvania.
Research informed by patients
Gene therapy, which has taken off in the last five years, involves injecting into a patient’s body a “vector,” a virus that cannot replicate but instead carries a copy of a gene to produce a working enzyme. The goal is to get the patient to produce, on his or her own, the missing enzyme whose deficiency is causing their disability.
CHOC is a growing destination for patients with rare diseases who receive gene therapy.
MPS 1 causes an abnormal build-up of various toxic materials, called glycosaminoglycans (GAGs), in the body’s cells. The disease affects about one in 100,000 newborns. MPS 1 is a spectrum of disease ranging from a mild form, called Scheie, to severe disease, called Hurler syndrome.
In his 16 years at CHOC, Dr. Wang has cared for 15 MPS1 patients. His youngest is 6 weeks old. Of these, 10 have Hurler syndrome; the youngest patient is 5 years old and the oldest is in his 20s.
”All of the research we do comes from the patients we see,” Dr. Wang says. “The question this study is trying to address came out of seeing my patients in clinic.
“Whether they’ve had a stem cell transplant or enzyme replacement therapy, our patients, who never missed a treatment, were complaining that their legs hurt or that they couldn’t put on clothes because their shoulders wouldn’t move the way they wanted them to.
“So the question arose that even though these kids were treated, how come their orthopedic symptoms weren’t getting better? They live with this every single day, which really does mess with their quality of life.”
Years in the works
Thus began Dr. Wang’s 12-year quest that first involved injecting enzyme into joints of dog with MPS I, work that was supported by the CHOC Specialists physician’s group, a study that showed promise in treating the orthopedic symptoms.
However, “patients aren’t super enthusiastic about the idea of having a joint injection repeatedly – it would work, but it wouldn’t be a very practical treatment,” Dr. Wang says.
Dr. Wang thought: What if we equipped the cells of the joints with the ability to make the enzyme on their own?
That way, he reasoned, an animal model could get a “one-and-done” injection. This thinking led to an important study examining gene therapy delivered to the joints of three MPS I puppies.
That study showed promise, with the joints making a lot of enzyme in the first few months after the injection, but after that the production dropped significantly.
Though not the home run he was looking for, Dr. Wang resolved to apply the lessons of the first gene therapy study by securing the R01 grant, which aims to determine which refinements to gene therapy will allow durable and full treatment of neurologic, orthopedic, and other MPS I symptoms in humans.
Challenging lives
Dr. Wang says his MPS 1 patients are functional – some have graduated from college – but daily life is particularly challenging.
“They wake up in the morning and they’re like, ‘Oh boy, this is going to hurt,’ but they still put one foot in front of the other. They have amazing families and are heroes in terms of how they live their lives.”
This is Dr. Wang’s first NIH grant. Assisting him in the study at CHOC will be Dr. Shih-Hsin Kan, who will be measuring the enzyme levels in the animal models.
“My role as the principal investigator,” Dr. Wang says, “is to make sure, like a conductor, that everyone is in sync.”
Learn about pediatric research and clinical trials at CHOC