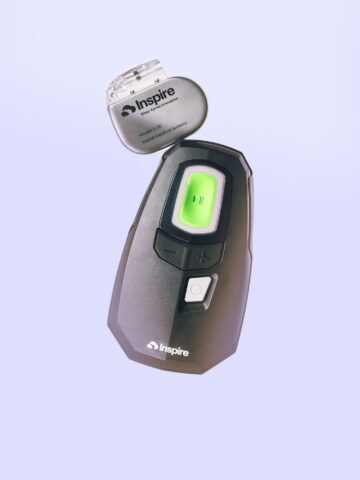
Tiny premature babies who suffer from a common but potentially fatal opening in their hearts are now being treated with a new device by physicians at CHOC.
The team successfully completed the first procedures March 20 and 21 to close a patent ductus arteriosus (PDA), an opening between two blood vessels of the heart that has failed to close on its own. CHOC became one of the first hospitals to use the Abbott Amplatzer Piccolo™ Occluder since it was approved by the FDA in January.

“We’ve never had the capability of doing this here at CHOC,” says Dr. Amir Ashrafi, director of CHOC’s neonatal-cardiac intensive care. “While closing a duct in a catheterization lab is not a new technology, closing a duct in a cath lab in very small babies is a big deal. The fact that we are now going to be one of the centers that are doing this, that is a big deal.”
PDAs are among the most common heart defects in premature babies. The opening, also called a duct or channel, is present in all fetuses and plays a vital role in allowing oxygen-rich blood from the mother to circulate through the unborn child’s body. In most cases, it closes spontaneously after birth. But out of the 60,000 infants born prematurely each year, 1 in 5 (12,000) has a PDA severe enough to require urgent medical attention.
“What happens is that blood goes in the wrong direction, so instead of blood going to the body, it goes into the lungs, so now the lungs get flooded,” Ashrafi says.
Without treatment, a PDA can cause breathing difficulty and a variety of other problems.
“It affects their feeding, because they’re having such a hard time breathing,” says Dr. Gira Morchi, an interventional pediatric cardiologist at CHOC. “It’s a cascade effect. It can affect the GI tract, kidneys, and the brain. Taking away the extra workload on the body allows for recovery.”
Abbott had previously developed the Amplatzer™ Duct Occluder to treat the same problem in larger pediatric patients. The new, smaller, device – measuring 3 mm by 2 mm – can be used in patients as young as 3 days old and weighing as little as 1.5 pounds, or 700 grams.
The procedure is performed through cardiac catheterization via a small incision made in the baby’s leg, near the groin area, to access a vein leading to the heart. A catheter is inserted, with the device inside. It’s the size of a small pea, and made of tightly woven metal mesh. Cameras and ultrasound guide the operator – in this case, Morchi – “correctly position the device” in the heart, she says. The device is deployed and placed in the opening, where it expands on its own. The device stays in the body, with tissue healing around it.
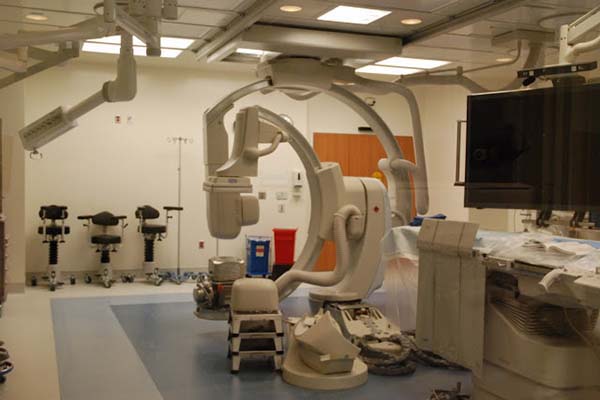
The first patient was a girl from Fullerton, Calif., who was born at 28 weeks and weighed 1.1 kilograms. The second patient, a girl from Huntington Beach, was born at 25 weeks and weighed 800 grams. The procedures were conducted when the babies were 2.5kg and 2.4 kg, respectively. The device is approved for much smaller infants than those, but the CHOC team is being selective about its cases.
“We’ll just slowly work our way down,” Ashrafi says.
Morchi herself has been doing such catheterizations for a decade. “We’re very comfortable with actually doing the procedure, so the real art here is to keep the babies stable while this procedure is happening,” she says. Conditions in the cath lab should closely match those in the NICU, including keeping the temperature warm. “We crank the heat up.”
Start to finish, the baby is in the room for 90 minutes to 2 hours, but the actual procedure only takes 20-30 minutes, she said.
Besides Morchi, the interventional cardiology team also included Dr. Sanjay Sinha and Dr. Mitch Recto.
The achievement was the result of a 2-year collaboration between CHOC and UC Irvine. Credit also goes to Dr. Evan Zahn of Cedars Sinai Medical Center in Los Angeles, who was an early adopter of the procedure and was lead investigator in the FDA approval study for the Abbott device. The trial included 50 patients at eight facilities in the U.S.
Upon FDA approval of this device, Abbot Vascular recognized CHOC as one of the first hospitals in America to use this device for those smallest and most vulnerable patients in the hospital.
“This device offers a new era in treating PDAs, and was successful at CHOC in great part due to a strong effort of collaboration between the cardiologist and the neonatologists,” says Dr. Sinha, a CHOC/UCI pediatric cardiologist.